KIPMIKIPMI
Communications in Science and TechnologyCommunications in Science and TechnologyThe oxidation of dipyrromethane by 2,3-dichloro-5,6-dicyano-1,4-benzoquinone (DDQ) generally produces dipyrrin, but in the presence of trace water, a meso-hydroxy dipyrromethane can be formed. To investigate this unusual product, we then studied meso-hydroxy bis(p-anisoyl)-p-fluorophenyl dipyrromethane (3) obtained from the oxidation of bis(p-anisoyl)-p-fluorophenyl dipyrromethane (2). Spectroscopic studies (1H-NMR, UV-Vis, and fluorescence), mass spectrometry, and computational analyses were performed to investigate this mechanism. Zinc complexation of compound 3 altered the 1H-NMR spectrum and shifted the absorption peak from 325 nm to 567 nm with “turn-on fluorescence. Thermochemical studies have indicated that the formation of meso-hydroxy requires energy higher than dipyrrin. This study suggests that the electronic properties of meso-aryl and acyl groups are the key factors for the nucleophilic attack of water on cationic dipyrromethane intermediate. These results further improve the understanding of dipyrromethane oxidation pathways, which is crucial for the design and synthesis of dipyrrin-chemosensors.
This study successfully synthesized and characterized meso-hydroxy acyl dipyrromethane, revealing its unique spectroscopic properties and complexation behavior with zinc(II).The research demonstrated that dipyrrin is thermodynamically more stable than meso-hydroxy dipyrromethane, explaining the observed reaction pathways.Furthermore, computational analyses elucidated the role of substituent effects in modulating the formation of meso-hydroxy dipyrromethane during dipyrromethane oxidation.
Penelitian lebih lanjut dapat dilakukan untuk mengeksplorasi pengaruh variasi substituen pada posisi meso dan diasil terhadap stabilitas dan reaktivitas meso-hydroxy dipyrromethane. Hal ini dapat dilakukan dengan mensintesis serangkaian senyawa analog dengan substituen yang berbeda dan menganalisis sifat-sifatnya secara komprehensif. Selain itu, studi mendalam mengenai mekanisme kompleksasi antara meso-hydroxy dipyrromethane dengan ion logam transisi lainnya, seperti tembaga atau nikel, dapat membuka peluang pengembangan sensor ion logam baru dengan sensitivitas dan selektivitas yang lebih tinggi. Terakhir, penelitian dapat difokuskan pada aplikasi potensial meso-hydroxy dipyrromethane dan kompleks logamnya dalam bidang-bidang seperti fotokatalisis, terapi fotodinamik, atau sebagai bahan aktif dalam perangkat optoelektronik, dengan tujuan memanfaatkan sifat-sifat unik senyawa ini untuk menciptakan teknologi inovatif.
| File size | 872.88 KB |
| Pages | 7 |
| DMCA | Report |
Related /
KIPMIKIPMI Co-pyrolysis menghasilkan PI yang menengah dengan memaksimalkan efisiensi dekomposisi melalui aksi sinergis bahan baku. Karena efeknya pada reaksi sekunder,Co-pyrolysis menghasilkan PI yang menengah dengan memaksimalkan efisiensi dekomposisi melalui aksi sinergis bahan baku. Karena efeknya pada reaksi sekunder,
KIPMIKIPMI Model pseudo‑second‑order dan model Peleg memberikan kecocokan terbaik dengan nilai R² tinggi. DES 70 % menghasilkan total flavonoid tertinggi sebesarModel pseudo‑second‑order dan model Peleg memberikan kecocokan terbaik dengan nilai R² tinggi. DES 70 % menghasilkan total flavonoid tertinggi sebesar
KIPMIKIPMI 4 nm to 14. 8 nm and surface areas from 27.69 to 31.67 m²/g. Despite having a lower surface area, T-Gm exhibits excellent photocatalytic efficiency (90.23%)4 nm to 14. 8 nm and surface areas from 27.69 to 31.67 m²/g. Despite having a lower surface area, T-Gm exhibits excellent photocatalytic efficiency (90.23%)
KIPMIKIPMI Pelapisan Cu pada paduan Al meningkatkan sifat antimikroba, ketahanan korosi, dan kekerasan material. Penelitian ini berhasil memproduksi lapisan tembagaPelapisan Cu pada paduan Al meningkatkan sifat antimikroba, ketahanan korosi, dan kekerasan material. Penelitian ini berhasil memproduksi lapisan tembaga
KIPMIKIPMI Penelitian ini bertujuan untuk menyelidiki peran nanokomposit zinc oxide/nickel oxide (ZnO/NiO) dalam meningkatkan efisiensi degradasi fotokatalitik molekulPenelitian ini bertujuan untuk menyelidiki peran nanokomposit zinc oxide/nickel oxide (ZnO/NiO) dalam meningkatkan efisiensi degradasi fotokatalitik molekul
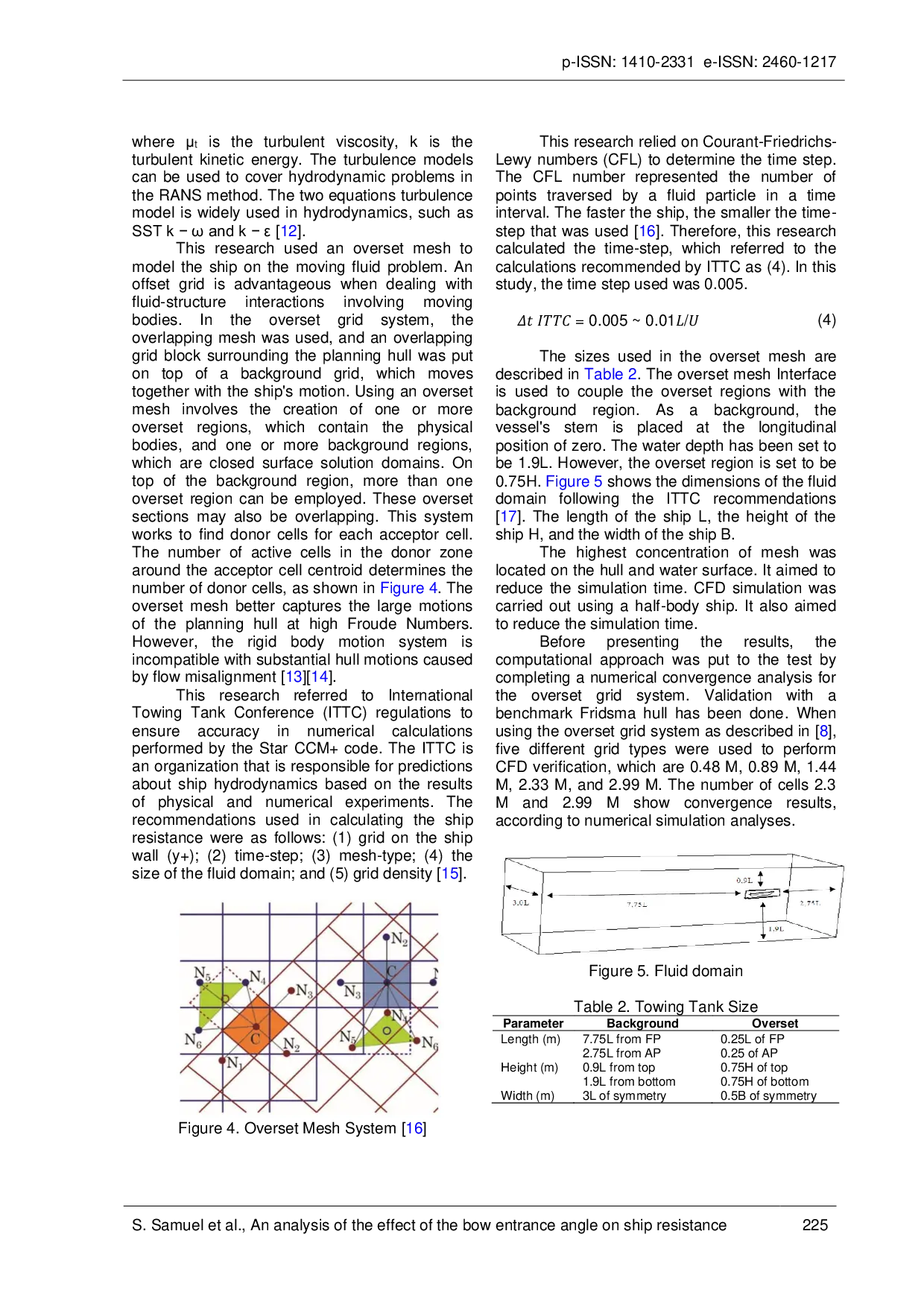
UMBUMB 67), a smaller bow angle reduces ship resistance, while at higher Froude numbers (Fr > 1), a larger bow angle is more effective. Ultimately, engineering67), a smaller bow angle reduces ship resistance, while at higher Froude numbers (Fr > 1), a larger bow angle is more effective. Ultimately, engineering
UMBUMB Nilai terkecil adalah peningkatan amplitudo goyangan dan yaw yang dipengaruhi oleh kondisi banjir satu atau dua tangki kosong yang berdekatan di bagianNilai terkecil adalah peningkatan amplitudo goyangan dan yaw yang dipengaruhi oleh kondisi banjir satu atau dua tangki kosong yang berdekatan di bagian
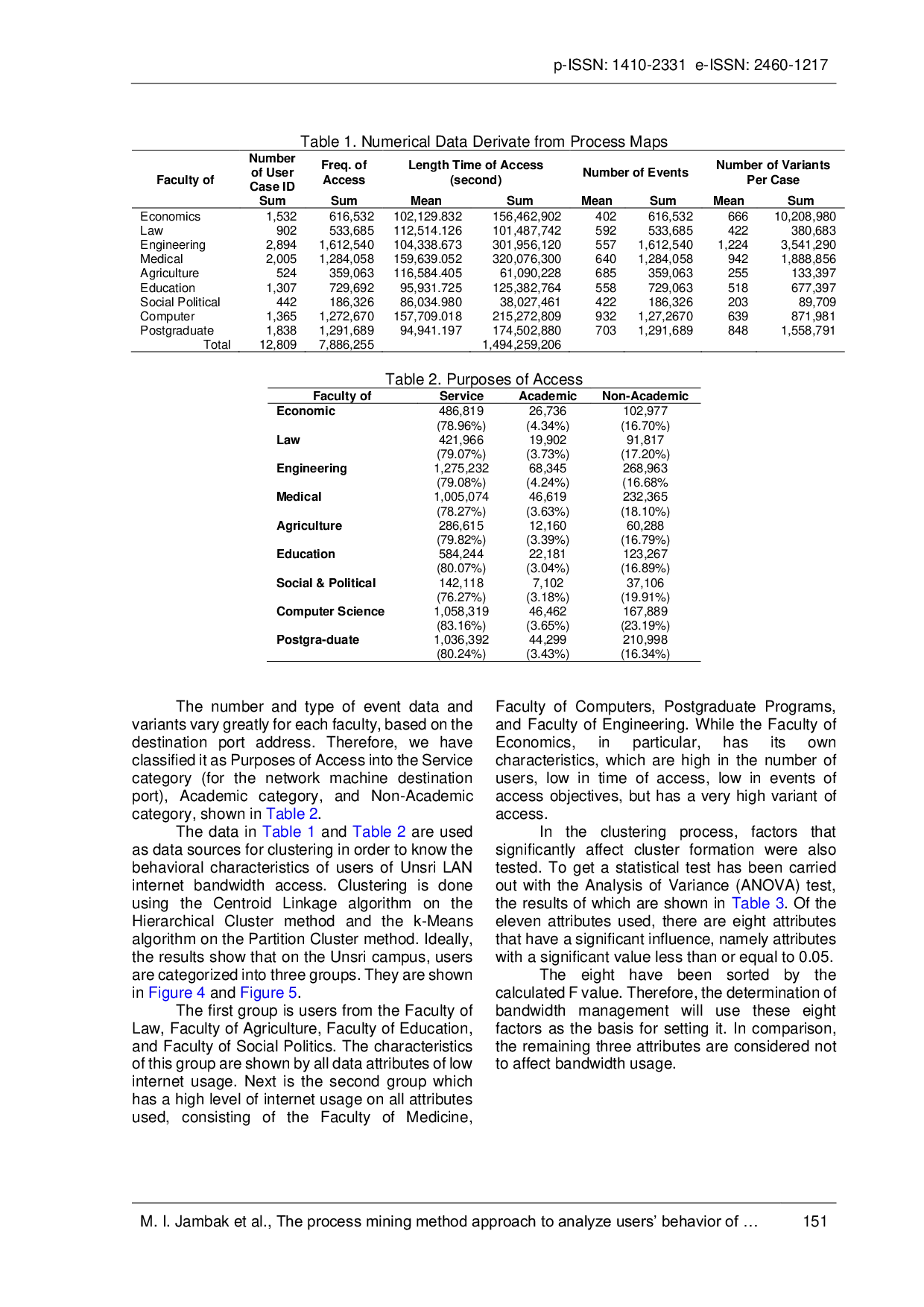
UMBUMB The Sriwijaya University internet network management unit does not yet have a standard formulation for implementing Bandwidth Management & Bandwidth Allocation.The Sriwijaya University internet network management unit does not yet have a standard formulation for implementing Bandwidth Management & Bandwidth Allocation.
Useful /
DIGLOSIA UNMULDIGLOSIA UNMUL Terdapat peningkatan rata-rata nilai siswa dari yang semula 64,7 (pra-siklus) menjadi 74,3 (siklus I) dan 83,2 (siklus II). Tingkat ketuntasan belajarTerdapat peningkatan rata-rata nilai siswa dari yang semula 64,7 (pra-siklus) menjadi 74,3 (siklus I) dan 83,2 (siklus II). Tingkat ketuntasan belajar
KIPMIKIPMI Metodologi ini mudah disesuaikan dan dapat direproduksi untuk penelitian atau tujuan operasional yang sebanding. Penelitian ini berhasil mengevaluasi penggunaanMetodologi ini mudah disesuaikan dan dapat direproduksi untuk penelitian atau tujuan operasional yang sebanding. Penelitian ini berhasil mengevaluasi penggunaan
UMBUMB Algoritma ini memiliki akurasi tertinggi sebesar 95,827%, skor F sebesar 0,958, dan waktu pelatihan terendah. Hasil ini menunjukkan bahwa metode ensembleAlgoritma ini memiliki akurasi tertinggi sebesar 95,827%, skor F sebesar 0,958, dan waktu pelatihan terendah. Hasil ini menunjukkan bahwa metode ensemble
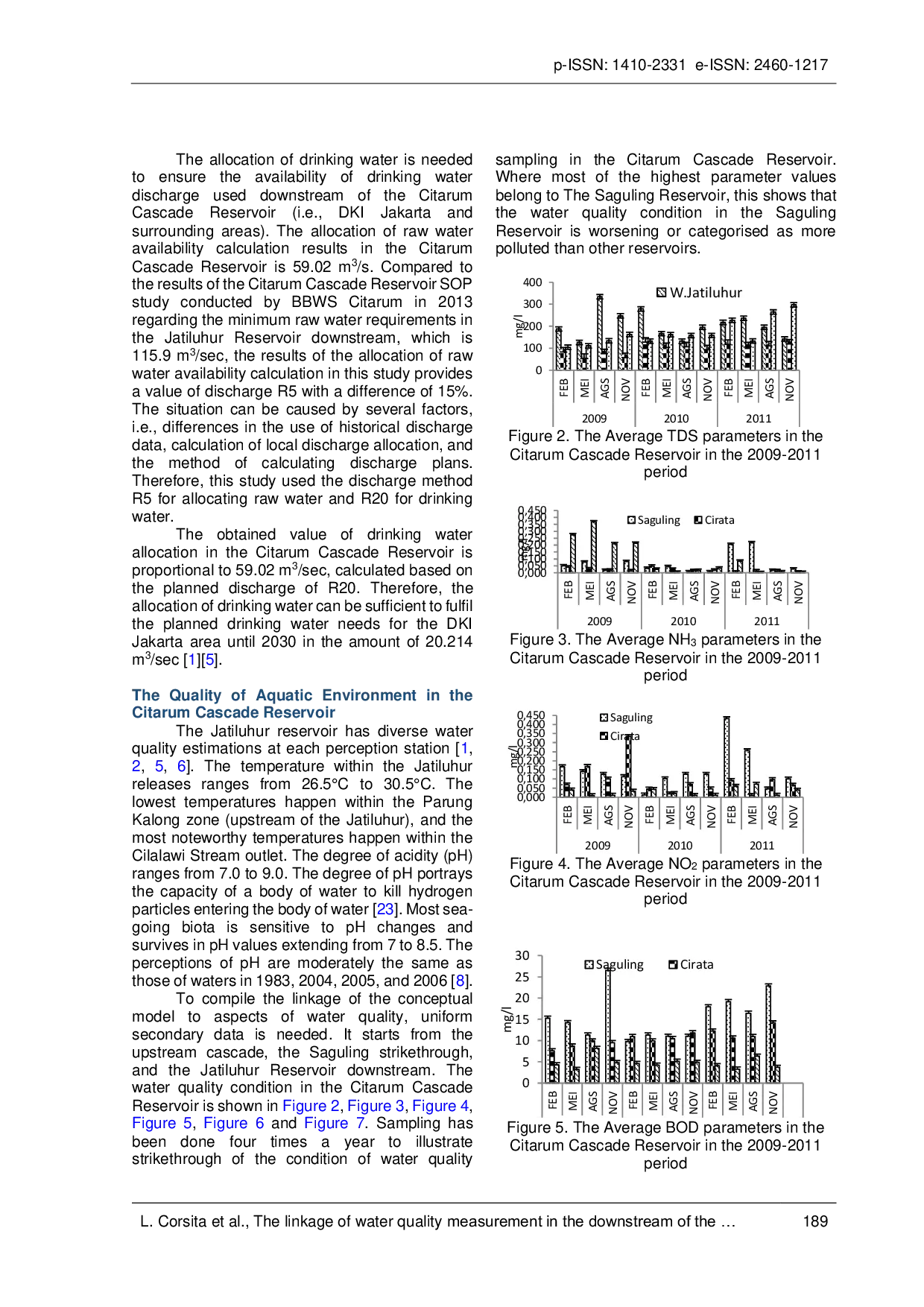
UMBUMB COD, BOD, and Pb, have exceeded the drinking water quality standard set by the Indonesian Ministry of health. The waters of the Citarum Cascade ReservoirCOD, BOD, and Pb, have exceeded the drinking water quality standard set by the Indonesian Ministry of health. The waters of the Citarum Cascade Reservoir